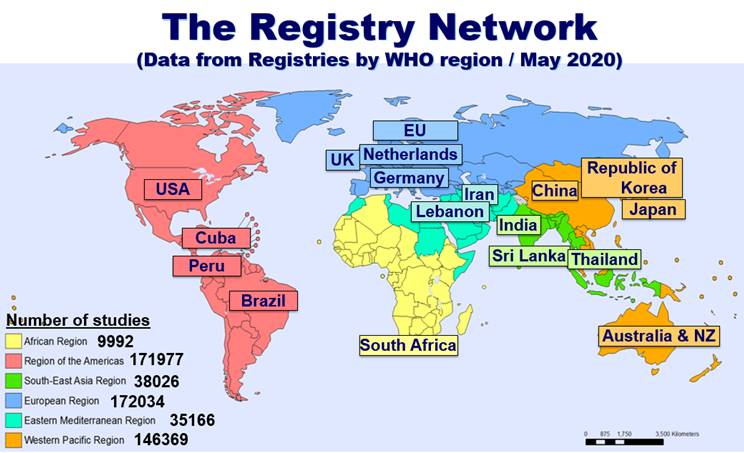
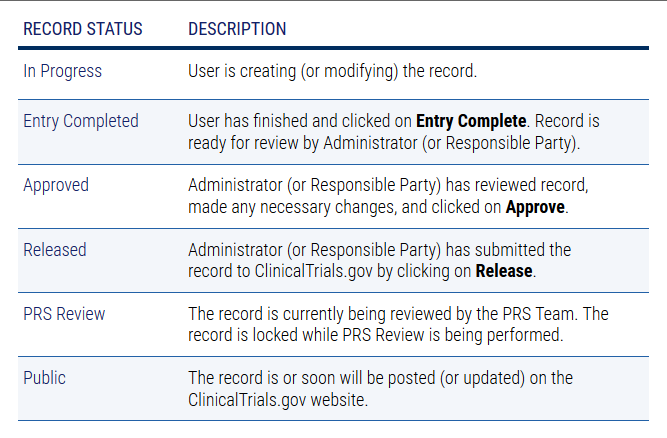
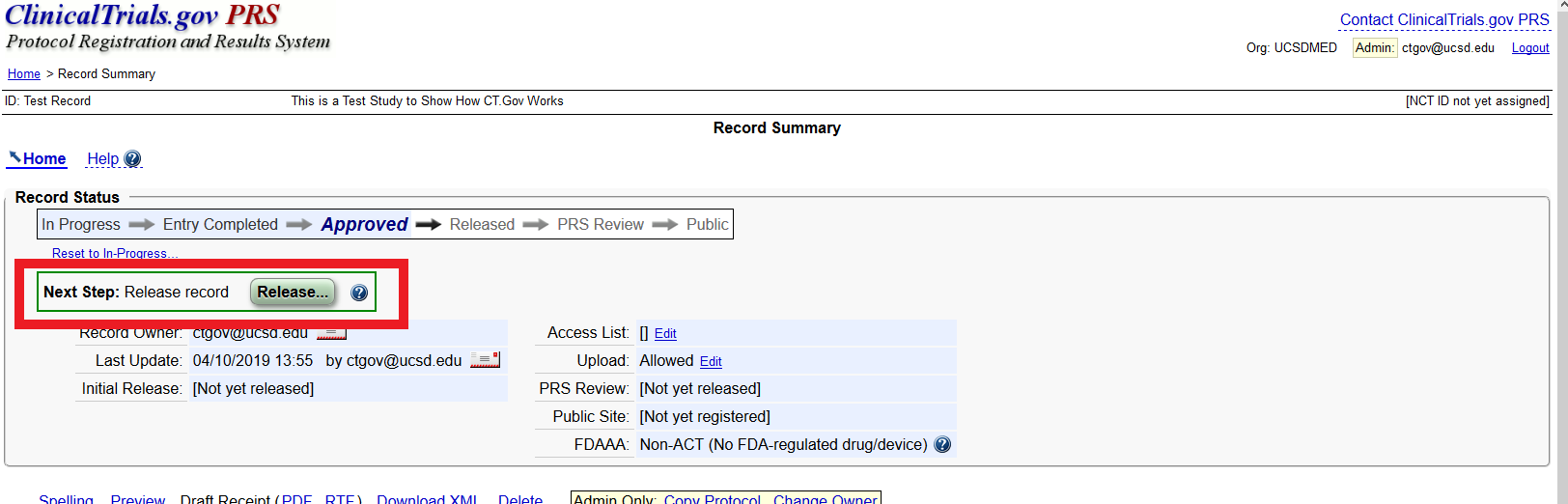
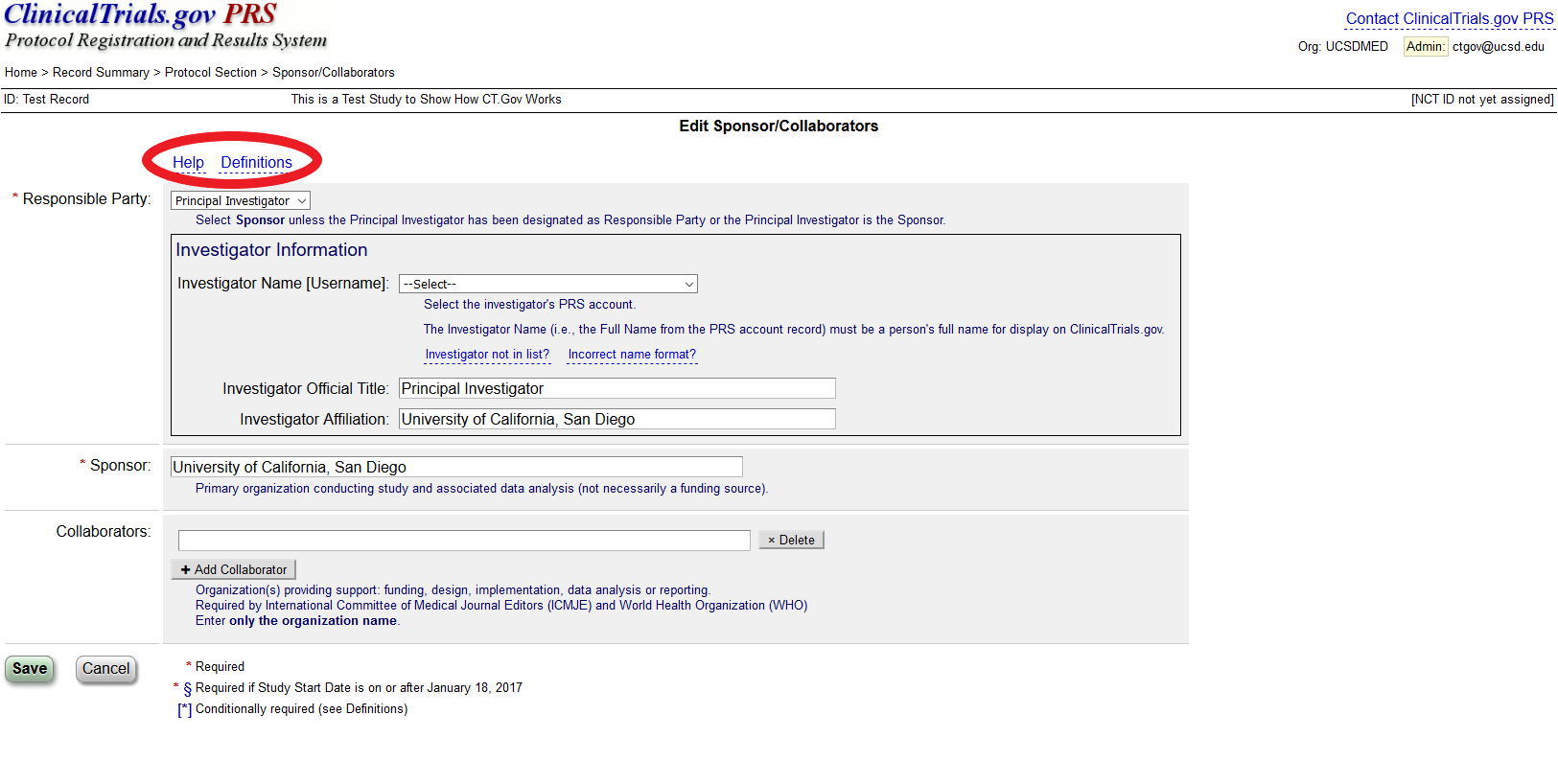
Don't Jeopardize Future Funding or Publication: Register and Upload Your Clinical Trial Data Now - Dean's Office Blog
Guidelines for Registering in the ClinicalTrials.gov Registry What Is ClinicalTrials.gov? Do I Need to Register My Clinical Tria

Publication of NIH funded trials registered in ClinicalTrials.gov: cross sectional analysis | The BMJ
Trial Publication after Registration in ClinicalTrials.Gov: A Cross-Sectional Analysis | PLOS Medicine